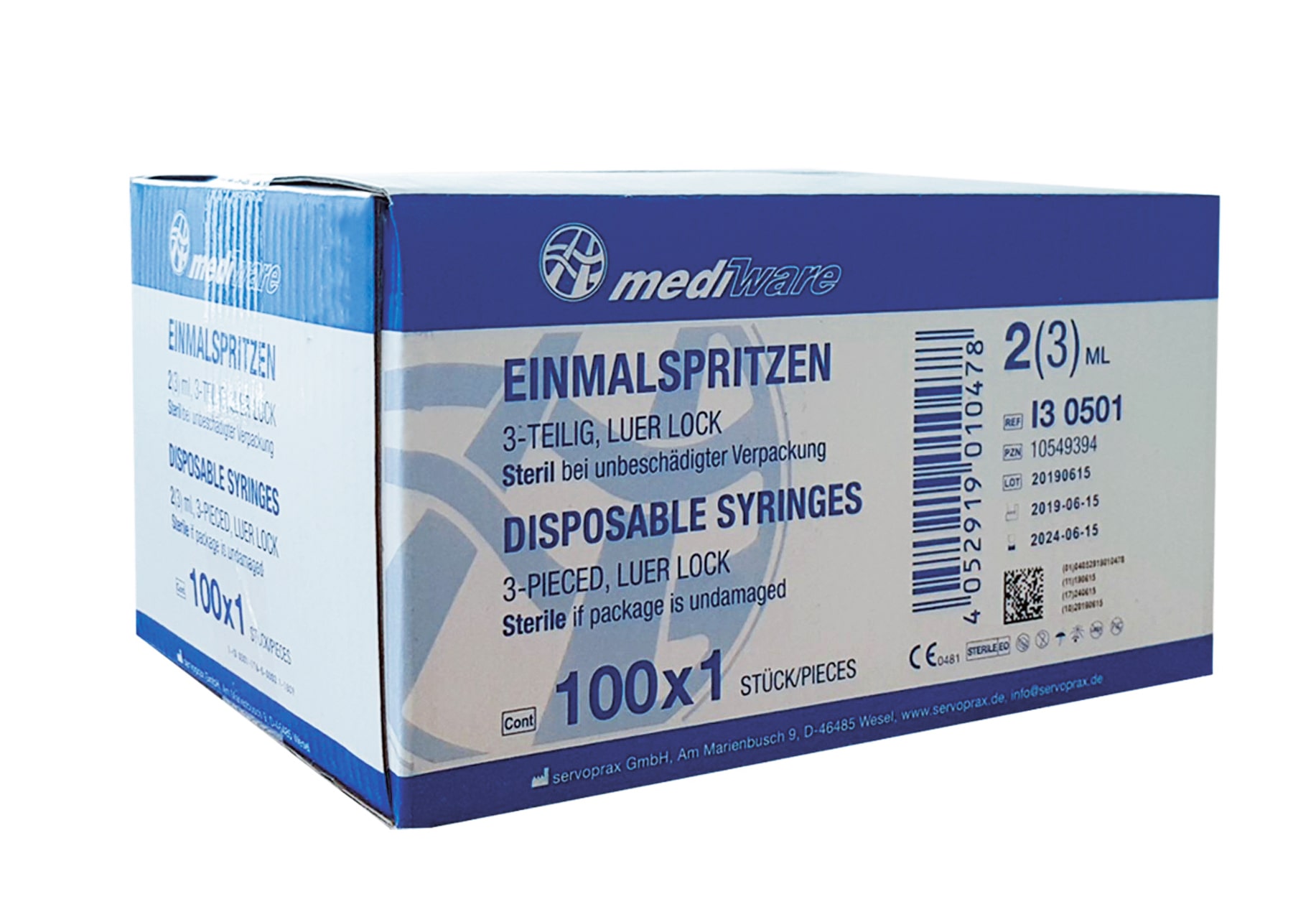
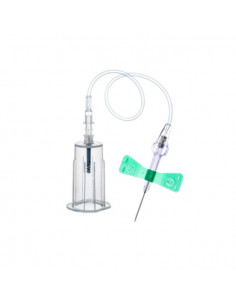
PRP Kit de démarrage | EBA 200
PRP Starter Set composé de :
1x centrifugeuse Hettich EBA 200
2x Tubes PRP | Vi PRP-Pro UE 10 pcs.
1x Aiguille KIPIC® 25G x 42mm, UE 100pcs.
1x Aiguille de mésothérapie KIPIC® 30G 4mm | UE 100 pcs.
1x Aiguille de mésothérapie KIPIC® 32Gx4mm | UE 100 pcs.
1x Mediware Seringues jetables 5ml 3-pièces Luer-Lock Stérile (UE 100 pcs.)
1x Kit de prélèvement sanguin de sécurité + adaptateur Luer 21G - UE 35 pcs.
1x Mediware Seringues jetables 2/3ml 3-pièces Luer-Lock Stérile (UE 100 pcs.)

RPmed.de : Votre expert en technologie PRP
Dans le domaine de la médecine esthétique, les traitements au plasma riche en plaquettes (PRP) gagnent en popularité. PRPmed.de est votre partenaire de confiance pour des produits et technologies PRP de haute qualité. Mais qu'est-ce que le PRP exactement, pourquoi est-il si demandé, et comment PRPmed.de peut-il vous soutenir de manière optimale ?
Qu'est-ce que le PRP ?
Le PRP signifie plasma riche en plaquettes – une méthode de traitement innovante qui utilise le sang du patient pour favoriser la régénération de la peau. Les applications sont nombreuses : le PRP peut être utilisé pour le rajeunissement de la peau ainsi que pour la restauration capillaire. Ce traitement naturel connaît un succès croissant en raison de son efficacité et de ses effets secondaires minimes.
PRPmed.de : Votre spécialiste en technologie PRP
PRPmed.de est votre boutique en ligne professionnelle, spécialisée dans la technologie PRP. Que vous soyez médecin, naturopathe ou professionnel dans le domaine de l'esthétique médicale, nous proposons une large gamme de produits PRP adaptés à vos besoins spécifiques.
Nos produits phares
L'un de nos produits phares est le tube VI - PRP-PRO PRP, conçu pour la préparation sûre et efficace du PRP. Nous proposons également une crème régénérative PRP innovante, qui peut être combinée avec le plasma du patient pour favoriser le renouvellement cellulaire et la régénération de la peau.
Pourquoi PRPmed.de ?
En plus de notre vaste gamme de produits PRP, nous nous distinguons par notre qualité et notre expertise exceptionnelles. Nos aiguilles d'injection KIPIC®, par exemple, sont idéales pour la mésothérapie, les injections de toxine botulique, et bien d'autres applications. Avec PRPmed.de, vous choisissez des produits qui répondent aux normes médicales les plus élevées.
Sécurité et service client
Votre sécurité est notre priorité absolue. C'est pourquoi nous proposons uniquement des dispositifs médicaux classés Risque IIa + b, garantissant une utilisation conforme à la législation en vigueur. De plus, nous soutenons les professionnels avec des formations spécialisées PRP, vous offrant la possibilité d'élargir vos connaissances et compétences en médecine esthétique. Notre équipe de service client qualifiée est toujours à votre disposition pour vous aider à choisir les produits adaptés à vos besoins.
Conclusion
Que vous soyez un praticien expérimenté ou un nouveau venu dans le monde de la technologie PRP, PRPmed.de est votre partenaire de confiance. Avec une large gamme de produits, une qualité supérieure, une expertise approfondie et un service client exceptionnel, nous sommes votre boutique en ligne de référence pour tous vos traitements liés au PRP.