- Home
- PRP
- PRF
- Laboratory equipment
- Injection
- dermal filler
- ANTI AGING
- hygiene
- carboxy
- Mesorollers
- PRP courses
- Medical training
- Online courses
- Online Course - Carboxy-Therapy
- Online Course - Classical Mesotherapy in Medicine & Aesthetics.
- Online Course - Microneedling PROFESSIONAL
- Botulinumtoxin A - online Kurs
- PRP in der Ästhetik, Trichologie und Orthopädie
- PRP therapy and its application in orthopaedics
- Hair Transplantation | Online Intensive Course
- PRP-Schulung in der Ästhetik
- Care
- Threadlifting
Profhilo H+L: Revolutionary skin rejuvenation through bio-remodelling
Profhilo H+L is more than just an ordinary skincare product. It is an innovative solution that can renew your skin's natural beauty from the inside out.
Discover the transformative power of Profhilo H+L:
- Bio-remodelling: Stimulates the skin's own collagen and elastin production to improve skin structure and elasticity.
- Long-lasting results: The unique formula of Profhilo provides long-lasting hydration and tightening of the skin.
- Versatile application: Profhilo can be used on various parts of the body to reduce wrinkles, lines, and sagging skin.
- Quick and easy treatment: The Profhilo treatment is minimally invasive and only takes a few minutes.
Content:
- 1 x 2ml syringe
- Original product by IBSA!

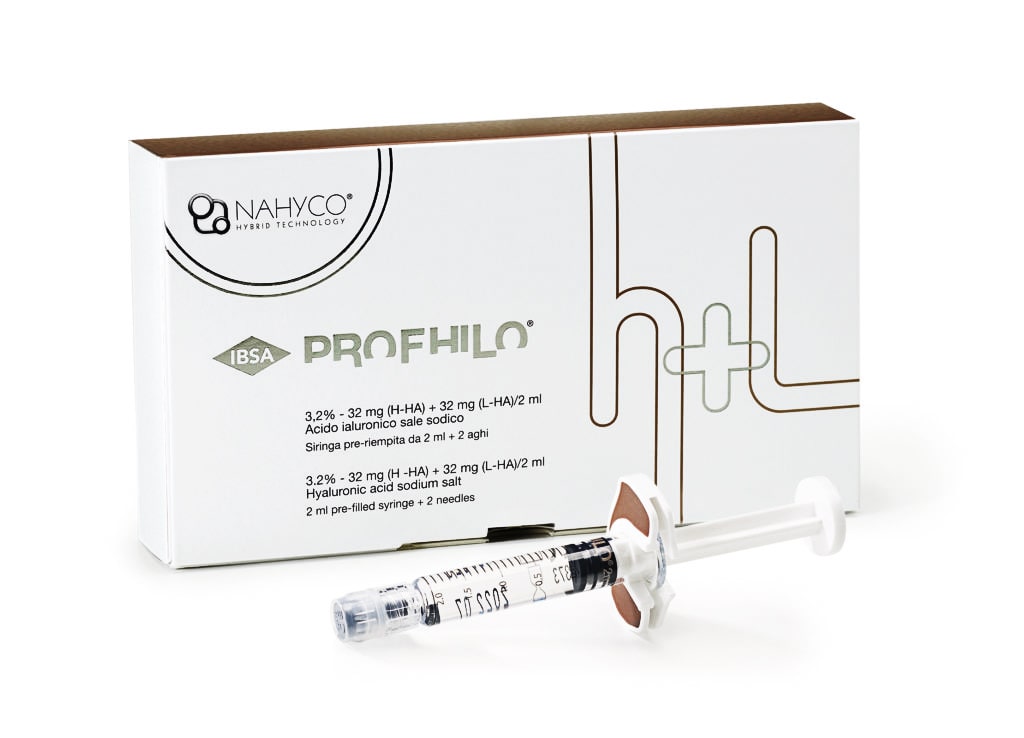
PROFHILO® - Bio Remodeling
PROFHILO H+L is the first stabilized hyaluronic acid (HA) based product manufactured without the use of chemical cross-linking agents (BDDE). It is used for skin reshaping and treatment of skin sagging, not only for filling lines and wrinkles.
Profhilo is a new trend in WOW aesthetics. It is a stable hyaluronic acid injection that can be used to treat sagging skin
Profhilo from IBSA is a highly purified hyaluronic acid. Through a patented bio-fermentation process, it is classified as "top quality" worldwide. The product is characterized by purity, safety and high resistance to thermal production processes.
PROFHILO is an innovative skin-firming preparation that enables multi-stage dynamic remodeling of the face, neck and body. Profhilo is a new anti-aging ally that nourishes the cells of the dermis and increases their elasticity, it has a long-lasting lifting effect.
Content 1x2ml syringe
The package contains 1 prefilled syringe with 2 needles type 29G x ½" (0.33 x 12 mm)
- 2 ml pre-filled syringe (32 mg (H-HA) + 32 mg (L-HA) hyaluronic acid sodium salt in 2 ml buffered isotonic saline);
Medical device for intradermal use
Sterile - single-use product.
PROFHILO® TREATMENT AREAS
Profhilo is not designed as a dermal filler. Therefore, it is not as effective to add volume, restrict movement or shape. Instead, Profhilo's formulation improves tone, texture and elasticity. The result is a healthier, younger-looking complexion.
It dramatically improves tissue quality, even in difficult areas, and complements other aesthetic treatments and dermal filler procedures. Its effect can last from 6 to 9 months.
Treatment with Profhilo
Profhilo can be used anywhere the skin needs to be rejuvenated, but most typically:
" tops of the cheeks
" side of the face
" Lines around the mouth
" Nasolabial folds
" Laugh lines
With only 5 small injections per side of the face, the most receptive BAP are injected.

It is an anti-wrinkle treatment that can turn back time and bring back natural beauty.
It is recommended that patients receive two treatments four to six weeks apart. Unlike many other dermal fillers and injections, Prophilo is safe for multiple applications throughout the body
The benefits of hyaluronic acid work well for general treatment areas such as the face, neck and arms, as well as the underarms. Abdomen and knees. The most common BAP card is designed to provide the best guidelines for precise positioning for specific measurements for the face and neck. For example: see the description below. Compared to other injection methods, there is a much lower risk of bruising, redness and inflammation at the injection site with the innovative BAP Profilo technology.
THE RESULT CAN BE SEEN WITH PROFHILO®!
The injection points spread over a wide area in the subcutis and immediately produce a fresh appearance. After PROFHILO® remains in the tissue longer, it stimulates intensive collagen formation.
Due to the special manufacturing process, PROFHILO® remains in the tissue 30 times longer than uncross-linked hyaluronic acid (as found in mesotherapy products), where it unfolds its moisturizing and stimulating effect. PROFHILO® contains 64 mg of hyaluronic acid per 2 ml of product, making it one of the most concentrated hyaluronic products on the world market.
PROFHILO® is injected with very fine needles at only 5 defined injection points per half of the face. Due to its special flowability, PROFHILO® distributes itself in the tissue. Immediately after the application, you can usually return to your usual daily routine
A treatment cycle consists of at least 2 applications at intervals of 4 weeks. A booster treatment is recommended after 4 to 6 months

PROFHILO® IS BIOCOMPATIBLE
Is Profhilo natural? Well, it is a non-surgical hyaluronic acid (it occurs naturally in our body), so it is not necessary to add any chemicals. It is not actually a dermal filler or mesotherapy
Traditionally, dermal fillers contain a synthetic chemical called BDDE. BDDE is short for 1,4-butanediol diglycidol, a magical ingredient that binds the entire hyaluronic acid chain to prevent it from breaking down in the body. It is naturally stable, so there is no need to add chemicals. Therefore, it is not actually a dermal filler or mesotherapy product, but a unique product unto itself
PROFHILO® has a unique and patented formula. When exposed to heat, high and low molecular weight hyaluronic acid combine to form a hybrid molecule. This thermal cross-linking eliminates the need for additives (such as BDDE), resulting in excellent product compatibility.
PROFHILO® is a high-purity hyaluronic acid that has a strong water-attracting effect that moisturizes the skin from within, stimulates the body's own hyaluronic acid production and promotes the formation of elastin and collagen.
PROFHILO® IS SAFE
The absence of additives ensures the best possible tolerability and minimizes the risk of side effects.
PROFHILO® can be combined with all common fillers, PRP, laser and botulinum treatments.
PROFHILO® WAS AWARDED!
Profhilo was awarded Product Innovation of the Year 2016 at the 2016 Aesthetics Awards in the UK and the International Anti-Aging and Beauty Trophy for Best Skin Improvement Product at the European Congress of Aesthetic and Anti-Aging Medicine in Paris in October 2015. in 2018, PROFHILO® was crowned "Best injectable Product".

PROFHILO® treatment is fast, gentle and hardly noticeable.
At five defined injection points on each side of the face, tiny amounts of PROFHILO® are injected with very fine needles. Thanks to its special properties and the special BAP (Bio Aesthetic Points) injection technique, PROFHILO® is distributed throughout the tissue. The entire facial skin benefits from its moisturizing effect
Immediately after the treatment, you can return to your usual daily routine without any problems
One treatment with two applications at intervals of 4 weeks is usually sufficient to achieve a visible anti-aging effect. After another 6 months, a booster treatment is recommended. Of course, PROFHILO® can also be applied to other areas of the body such as the décolleté, neck and back of the hands. Where the skin is tired and dry, PROFHILO® brings moisture and thus new freshness to the tissue.

The BAP treatment techniques for face and neck
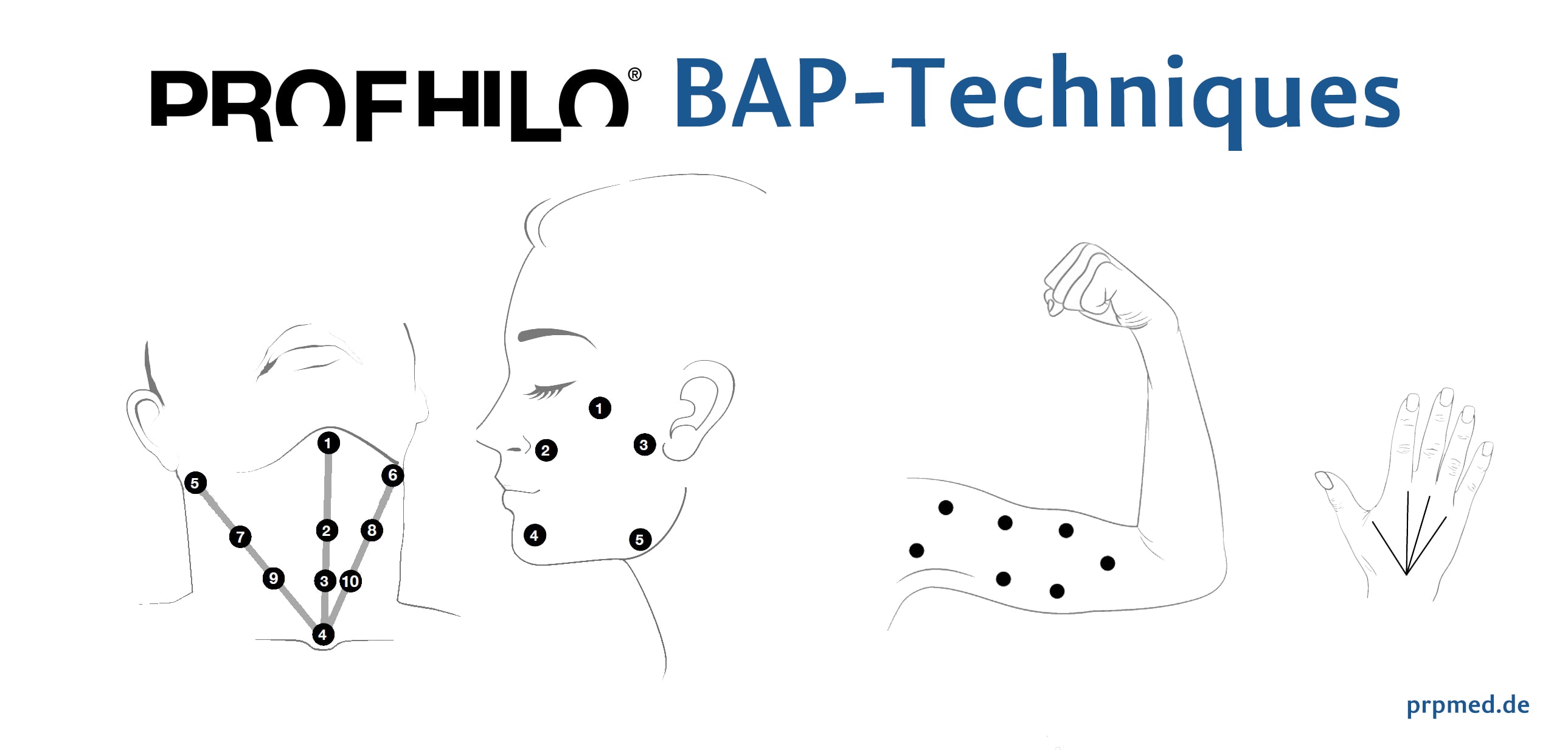
The BAP technique (BAP = Bio Aesthetic Points)
This technique makes it possible to flood the entire face with PROFHILO® with only 5 small injections per half of the face using the finest cannula (29G TW).
The dosed amount of the particularly absorbent BAP points allows the wide diffusion of PROFHILO® into the subcutaneous tissue and creates a natural and long-lasting effect for a fresh appearance. PROFHILO® can be combined with all standard cosmetic procedures (fillers and Botox).
Profhilo is intended for remodeling of tissues and elimination of atony of the skin of the face, neck, décolleté, the inner part of the shoulders and hands. The BAP technique (bioesthetic points) is used for the introduction of the drug
Each point is a specific area without large vessels and nerves, which is selected taking into account the anatomical structure.
Injections are administered at 5 points on each side of the face, i.e. only 10 injections are necessary.

Originally, the BAP technique was developed for the malar and submalar areas, as they are prone to skin atrophy due to aging. It is the most widely used and recommended protocol for the treatment of these areas.
Due to the high fluidity of PROFHILO®, without leaving irregularities in the tissue, a special BAP technique was developed for the neck.
This injection technique minimizes the risk of side effects in the form of papules, bruising and edema and also ensures that the drug is distributed as evenly and homogeneously as possible.
Profhilo application on the face to demonstrate the BAP technique
Profhilo application on the neck to demonstrate the BAP technique
The IBSA recommends 2 sessions at one month intervals using the BAP (Bio Esthetic Points) technique to minimize the risks and maximize the flowability of the product.
Five points for intradermal administration of PROFHILO® must be identified as follows:
1.Zygomatic prominence. It is recommended to maintain a distance of at least 2 cm from the lateral canthus (outer corner of the eye).
2.Base of nose. A line connecting the nostril and tragus and a perpendicular line extending from the pupil must be drawn to locate the injection site at the intersection of the two lines.
3.Tragus. It is recommended to stay at least 1 cm anterior to the lower edge of the tragus.
4.Chin. A vertical line at the center of the chin and a vertical line one-third of the distance from the top of the vertical line should be drawn. From the point of intersection, one should move 1.5 cm toward the corner of the mouth to find the injection point.
5.Lower jaw angle. The fifth point is located 1 cm above the angle of the mandible.
Then, 0.2 ml of the product must be injected into the deep dermal/subcutaneous levels using the bolus technique.
The injections should be followed by a gentle massage.
INDICATIONS
Through its corrective/filling action on natural and induced depressions of the skin, PROFHILO® applies:
" in the physiological process of skin aging, the effects of which include a decrease in skin hydration and changes in elastic fibers and skin collagen, accompanied by a loss of plumpness and tone;
" in the process of dermal tissue repair, which starts in the case of scars caused by superficial injuries of the skin (e.g. acne and pockmarks).
The viscoelastic and moisturizing properties of hyaluronic acid, combined with the maintenance of an adequate concentration of hyaluronic acid in the skin tissue, rehydrate the skin and create optimal conditions to prevent and counteract skin aging, while at the same time promoting tissue remodeling and the consequent corrective effect on the damage caused by photoaging and aging of the skin, as well as on any kind of scarring
And aging processes of the skin, as well as on any kind of scarring.
HYALURONIC ACID
Hyaluronic acid also plays a role inside the extracellular matrix, creating the physiological conditions for thriving, migration and organization of dermal cell components. Furthermore, the intradermal administration of PROFHILO® and its action at the level of the dermis instead of the epidermis allow the optimal amount of hyaluronic acid to be introduced directly into the tissues in need of treatment, where it counteracts the cytotoxic effect of free radicals on fibroblasts and the underlying fat layers, thus ensuring the effectiveness of preventive and corrective beauty treatments.
The hyaluronic acid used in PROFHILO® is obtained from a natural substrate by biosynthesis without further chemical modification. Therefore, PROFHILO® has excellent biocompatibility and its application in the dermis allows integration with endogenous hyaluronic acid, which has been reduced and modified due to physiological aging processes of the skin or as a result of superficial skin injuries.
In addition, in vitro studies were performed to determine any incompatibilities and/or interactions between PROFHILO® and Platelet Rich Plasma (PRP). The results obtained show that PRP does not cause any change in the rheological behavior of sodium hyaluronate.
PROFHILO® is indicated for treatments to redefine facial and body contours and to model sagging areas. However, it is particularly indicated for the treatment of the area on and under the cheekbone, upper arm, abdomen and other areas affected by sagging skin.
An initial cycle consisting of two treatments 30 days apart is recommended, which can be followed by maintenance treatments 2 months apart if necessary. In any case, it is recommended to evaluate the specific PROFHILO® protocol in adaptation to the individual skin aging degree of the treated person.
WHAT IS PROFHILO®?
Currently, hyaluronic acid (HA)-based dermal fillers are the most popular dermo-aesthetic medicine intervention for the treatment of skin aging. Recent studies have shown that the combination of HA chains of different lengths and molecular weights improves tissue repair and regeneration through a synergistic mechanism. Profhilo® is a product that has been on the market since 2015 and is based on stable, hybrid and cooperative complexes (HyCoCos) produced using NAHYCO® Hybrid Technology, an innovative thermal process that eliminates the use of chemical reagents. The result is a filler with high biocompatibility and low viscosity that promotes optimal diffusion at the tissue level to achieve the desired bioremodeling of the facial contour. The objective of this review is to provide data from the entire postmarketing experience after 3 years of use and more than 40,000 patients treated with the medical device.
It is the world's first product that combines lifting effect and moisturizing in one!
Launched in 2015, Profhilo® is a novel HA preparation from IBSA, based on stable hybrid cooperative complexes (HyCoCos) and is the first product developed using NAHYCO® hybrid technology, an innovative thermal production process patented by IBSA.
The package contains 1 prefilled syringe with 2 needles 29 G × ½ (0.33 x 12 mm) with the following available volume: -2 ml prefilled syringe - 32 mg (H - HA) + 32 mg (L - HA) hyaluronic acid sodium salt in 2 ml buffered physiological sodium chloride solution. The prefilled syringes are sterilized by moist heat and the needles are sterilized with ethylene oxide.
The manufacturing process starts with a simple mixture of 32 mg of high molecular weight HA (1100-1400 kDa) and 32 mg of low molecular weight HA (80-100 kDa). The mixture is then stabilized by the above-mentioned thermal process, which does not use crosslinking agents and consists of an initial high-temperature step followed by a low-temperature step.
The result is a product that promotes both tissue remodeling and repair processes, even when scars have already formed. It also improves skin sagging on the face, neck and body.
PROFHILO® is suitable for the treatment of the face and body, especially for the treatment of the malar-zygomatic and submalar areas. An initial cycle of two treatments 30 days apart is recommended, followed, if necessary, by maintenance treatments every 2 months. However, it is recommended to evaluate the specific PROFHILO® protocol according to the patient's aging level.
Important Notice:
As the seller, we point out that the injection of products with and without lidocaine should be performed only by trained and medically qualified personnel, medical and nursing.
THE INTRADERMAL INJECTION MUST ONLY BE PERFORMED BY A DOCTOR
PROFHILO COMPONENTS
Hyaluronic acid (HA) is a naturally occurring polysaccharide in the human body whose primary function is to maintain proper tissue hydration, made possible by its inherent ability to bind large amounts of water.
The sodium salt of hyaluronic acid is composed of repeated disaccharide units of N-acetylglucosamine and sodium glucuronate chains, and is a fundamental component of the extracellular matrix in much of tissue, including skin.
PROFHILO® is a buffered isotonic solution of high molecular weight (H-HA) and low molecular weight (L-HA) hyaluronic acid (HA).
The hyaluronic acid used in this product, both high and low molecular weight, is obtained through a process of biofermentation without chemical modification and therefore proves to be excellently tolerated. Furthermore, thanks to a special patented preparation of the solution (NAHYCO® Hybrid Technology), the H-HA and L-HA chains contained in PROFHILO® interact with each other, creating unique rheological characteristics that allow the administration of higher concentrated HA without an increase in viscosity
The formulation of hyaluronic acids with different molecular weights contained in PROFHILO® is based on the Hydrolift® Action. This innovative approach aims to counteract the physiological decline in the skin's hyaluronic acid content, restore hydration, elasticity and tone by synergistically combining deep hydration with the mechanical action of lifting the skin.
The other product components are: Sodium chloride, sodium phosphate and water for injection.
BUY PROFHILO?
Many customers ask, "Is Profhilo worth buying?" The simple answer is yes, it is worth it. It's fast, effective, minimally invasive and doesn't carry the same risks as other procedures.
Through Profhilo, you can offer your patients healthy, smoother, fresher-looking skin and a radiant appearance for years to come.
You can purchase professional Profhilo cosmetics from us at an attractive price. We guarantee the authenticity and high quality of the products.
In the online store https://prpmed.de/en/ you can quickly and easily order elite products for skin, body and hair care online.
We deliver worldwide
FAQ
PROFHILO® is not a filler or skin booster. Fewer sessions are required and it donates more afluid.
PROFHILO is not a filler, but a hyaluronic acid gel with innovative bioremodeling properties. It forms four types of collagen and elastin, which makes it an effective treatment for sagging skin, lack of moisture and tired skin in general.
PROFHILO solves the problem of dehydration and gives the skin back its natural glow.
It has already been stated that Profhilo offers similar benefits but is not a skin booster
Another popular treatment that many people confuse with Profhilo is exactly the same as Dermal Fillers. It is a bio-remodeling treatment that rejuvenates the natural elastic and collagen product in the skin
While fillers are ideal if you only want minor adjustments and improvements to certain areas of your face like cheeks or under the eyes, etc., Profhilo is the cosmetic facial you should choose if you want more comprehensive improvements to your face.
Profhilo falls into the category of "injections", but is very different from and not a substitute for hyaluronic fillers and Botox.
This treatment cannot compensate for the loss of facial volume or relax the muscles. The improvement in skin quality provides an extremely natural look.
It is an excellent first step in anti-aging.
Profhilo is made from the purest form of hyaluronic acid (HA), which is strategically injected into the top layers of the skin using a very small needle. It spreads effortlessly throughout the skin, where it has a "restorative" effect on the tissues, deeply hydrating them and initiating their biological remodeling process. Unlike dermal fillers, which are used to increase volume, Profhilo is used to improve skin quality.
The product remains on the skin for about 28 days, during which time it stimulates collagen and elastin production through the slow release of hyaluronic acid. Therefore, after 4 weeks the results become more visible and after 8 weeks you will notice that your skin looks young, hydrated and radiant.
Profhilo triggers a tissue reaction known as"bioremodeling" that smoothes, tightens and gives skin an inner glow. In short, Profhilo reshapes sagging skin and fights imperfections.
It is not a replacement for fillers and Botox and can be used in combination with these other treatments or as a stand-alone treatment.
Profhilo acts on the skin quality and not on the muscle movements that cause wrinkles (muscle relaxation injections treat these) or on the loss of volume of fat pads and the filling of certain wrinkles (these problems are treated with dermal fillers).
PROFHILO helps repair and rejuvenate the skin, tightens and gives your face a defined look, eliminates fine lines, expression lines and wrinkles, and smoothes the skin while eliminating wrinkles and sagging skin.
Anyone who shows signs of aging can receive Profhilo. The preferred age group for PROFHILO is between 40 and 50. But people outside the above age group can also use PROFHILO as a preventive treatment to keep their skin strong and in good condition.
It only takes two treatments a month apart to experience the benefits of PROFHILO on your skin, as you may see an effect after just one or two days, depending on your skin type. For others, the effects may not be visible for a week or more. However, the best results are usually seen 8 weeks after the first treatment.
On the face, only 5 injection points are required on each side of the face for optimal results.
It is injected just below the skin's surface, where it spreads quickly (since it lacks the cross-linking agent BDDE) and reacts with the skin's own tissues to immediately increase moisture levels.
In Phase 2, during the next 4 weeks, the production of collagen and elastin in the dermis is increased, which helps the skin achieve a youthful, smooth, soft and firm appearance.
The results of the treatment last up to six months.
Profhilo® can be used as a stand-alone treatment or in conjunction with other dermal fillers, laser treatments or Botox as part of a holistic approach to skin remodeling and revitalization.
In the production of Profhilo, a complex of high and low molecular weight acids is heated, causing their molecules to stick together. The mixture is then rapidly cooled. This process creates hydrogen bonds, leaving a high concentration of acid in the skin. Another advantage of Profhilo is that these compounds are made without additional chemical binders that could be toxic.
This means this product is safe and does not cause allergies or tissue rejection.
Profhilo is very successful in the correction of the neck and décolleté, as well as the inner surfaces of the forearms and hands. For the appearance of Profilo in the process of aesthetic neck rejuvenation was not without a rehabilitation period.
After Profilo injection, there is only slight redness, which subsides after a few hours. Due to the peculiarities of its production, the product has a low viscosity. The fluidity of Profilo allows the product to pass through the thinnest needle (leaving no residue on the skin) and spread almost immediately in the subcutaneous tissue.
Above all, this ensures a short procedure (generally no more than 15-30 minutes) and freedom from pain (no anesthesia required). In addition, after the injection of the drug on the skin quickly absorbed papules (about an hour and a half), which are an indispensable attribute of other cosmetic injections. In principle, the procedure can be performed even during the lunch break.
Thanks to the innovative injection technique used in a Profhilo treatment, only 5 small injections are required on each side of the face, making the treatment relatively quick and convenient.
Because Profhilo is injected directly under the skin with very fine needles at predetermined points, it is relatively painless; you may feel a brief stinging or scratching sensation, but this does not last long.
Patients may opt for a topical pain reliever or anesthetic prior to Profhilo® treatment, but this is generally not necessary. The procedure is very quick and only takes about 10 minutes.
Immediately after the procedure, small bumps will be seen at the injection site. These are usually very easy to cover and can take up to 24 hours to heal.
Contraindications include patients with bleeding disorders, immunocompromised individuals, pregnant women and individuals with unrealistic expectations. Book your personal consultation to find the best treatment for you.
Profhilo® results can last between 6 and 12 months, depending on your individual response and how the product is metabolized
It is recommended to come in for a one-time maintenance treatment every 3 months. This will keep your skin in exceptional condition.
Do not touch your skin. Avoid harsh skin care products such as retinols and AHAs that evening, we recommend no strenuous exercise for 48 hours
Anything that makes your face red (sports, steam baths, sun) will make you more prone to bruising and swelling.
Pregnant or breastfeeding women and anyone who has an infection at the injection site. Also, anyone who has already had an allergic reaction to a product, which is very, very rare. It is important that you discuss your medical history in detail with your doctor so that he can evaluate whether the treatment is suitable for you or not.
Collagen and elastin are frequently mentioned in this post and in relation to skin health. You may be wondering why collagen and elastin are so important?
Collagen is the protein that makes up the largest percentage of your skin, about 75-80%. Collagen plays a crucial role in strengthening the skin and can actually contribute to elasticity and hydration.
Elastin is another important protein that, as the name suggests, is incredibly elastic and is found in connective tissue. It helps the skin return to its original shape and position when pinched and stung. When elastin decreases, elasticity decreases and the skin does not correct the problems that younger skin would.
After biorevitalization, you can return to your usual lifestyle. However, UV light accelerates the breakdown of hyaluronic acid and inhibits the body's own collagen synthesis. Therefore, if you want to preserve the effect for as long as possible, you should protect your skin from sunlight.
Below are some tips to optimize the results of your treatment:
- Avoid alcohol the day before your treatment. This will help you minimize the risk of bruising.
- Avoid taking aspirin, ibuprofen or other anti-inflammatory medications the week before treatment, as this also increases the risk of bruising.
- If you are uncomfortable with a cold virus, cold sores (herpes), or other skin rash or condition, please call the clinic to see if you still need to keep your appointment. Your general health is very important for the treatment.
- After treatment, carefully follow the aftercare instructions given to you by your doctor and do not apply anything else to the treated area unless previously discussed.
Common side effects of Profhilo are similar to other types of fillers and include mild bruising and swelling. Risks are the same as with other fillers and may include allergic reactions or infections, although these are extremely rare.
Profhilo is ideal for combining with traditional fillers containing stabilized hyaluronic acid for more precise and subtle volume correction
Profhilo treatment costs 250€ - 500€
Profhilo is injected according to a standard pattern where 2 millimeters are distributed over 10 injection points, so the cost does not depend on the amount used.
The price you pay depends largely on the experience and location of your doctor's office.
Yes, Profhilo is worth it, but you need to find out if it is the best treatment option for your specific facial and skin goals. Profhilo is suitable for any skin type and will improve the aesthetics of any face. For dramatic changes in sagging skin or deep wrinkles, it is best to combine it with another treatment.
The special thing about profhilo is that expectations must be realistic, that is the key. If someone thinks that their face will be miraculously lifted, they are wrong: this is where fillers, threads or a treatment come into play. Just thinking about the quality of the skin, the glow of the skin and the hydration of the skin. It is great because it is an injectable moisturizer!
Customers who bought this product also bought:
Shine Brighter Microneedling Serum - Safe, effective and radiantly beautiful.
VACUETTE® EVOPROTECT safety blood collection set + holder 21G x 3/4" tubing length 12"...
Save products on your wishlist to buy them later or share with your friends.