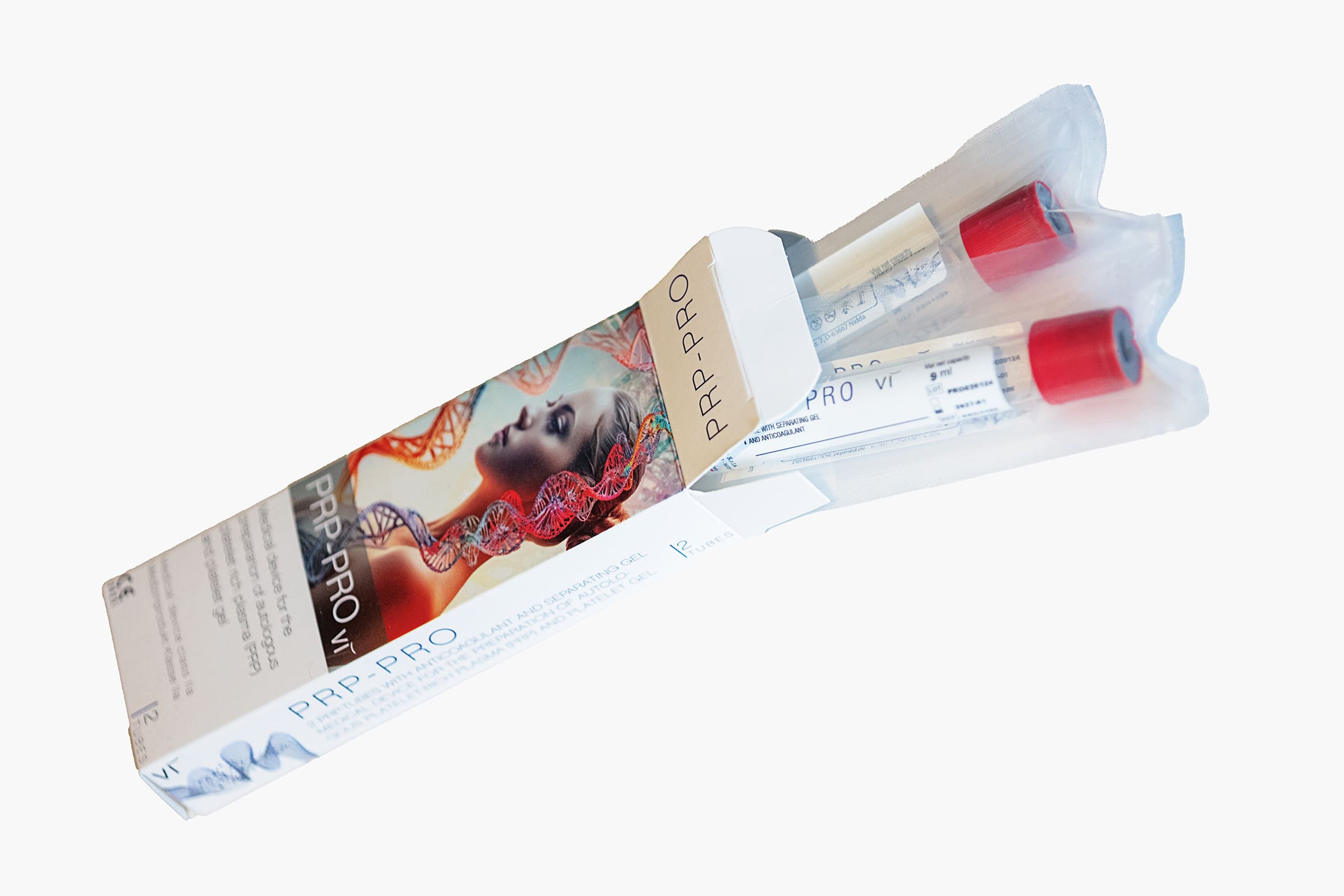
PRP tubes | Vi PRP-Pro | PU 10 pcs.
Vi PRP-Pro PRP tubes provide the simplest and most efficient method for obtaining and preparing platelet-rich plasma (PRP) from the patient's blood.

PRPmed.de: Your Expert in PRP Technology
In the field of aesthetic medicine, treatments with platelet-rich plasma (PRP) are becoming increasingly popular. PRPmed.de is your trusted partner when it comes to high-quality PRP products and technologies. But what exactly is PRP, why is it in such high demand, and how can PRPmed.de support you in the best possible way?
What is PRP?
PRP stands for platelet-rich plasma — an innovative treatment method that uses the patient's own blood to promote skin regeneration. The applications are diverse: PRP can be used for both skin rejuvenation and hair restoration. This natural treatment is gaining popularity due to its effectiveness and minimal side effects.
PRPmed.de: Your Specialist in PRP Technology
PRPmed.de is your professional online shop, specializing in PRP technology. Whether you are a physician, naturopath, or professional in medical aesthetics, we offer a wide range of PRP products tailored to your specific needs.
Our Top Products
One of our top products is the VI - PRP-PRO PRP Tube, designed for the safe and effective preparation of PRP. In addition, we offer an innovative regenerative PRP cream, which can be combined with the patient's own plasma to promote cell renewal and skin regeneration.
Why PRPmed.de?
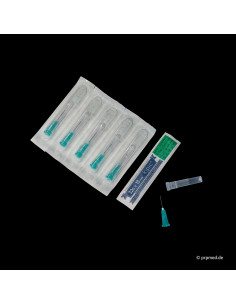
In addition to our comprehensive range of PRP products, we stand for outstanding quality and expertise. Our KIPIC® injection needles, for example, are ideal for mesotherapy, botulinum toxin injections, and many other applications. Choosing PRPmed.de means choosing products that meet the highest medical standards.
Safety and Customer Service
Your safety is our top priority. That’s why we only offer medical devices classified as Risk Class IIa + b, ensuring legally compliant use. We also support professionals with specialized PRP training programs, providing you the opportunity to expand your knowledge and skills in aesthetic medicine. Our knowledgeable customer service team is always available to help you select the right products for your needs.
Conclusion
Whether you're an experienced practitioner or new to the world of PRP technology, PRPmed.de is your reliable partner. With a broad product range, top-quality items, deep expertise, and excellent customer service, we are the online shop of choice for all your PRP-related treatments.
GPSR – General Product Safety Regulation
Responsible Economic Operator according to GPSR:
prpmed Funkner e.K.
Kurstraße 7
63667 Nidda
Germany
Phone: +49 6043 9862 817
Email: kontakt@prpmed.de